A cancer diagnosis often brings a problematic journey, harsh treatments, invasive surgeries, and an uncertain future. But for a small group of rectal cancer patients, a clinical trial delivered an outcome no one saw coming. Every single participant walked away cancer-free.
The results left oncologists stunned. Not a trace of cancer remained in scans, not in biopsies, not even under the most rigorous medical scrutiny. Such a complete remission had never been documented in a trial like this.
The Trial That Shocked Experts
Eighteen patients, all diagnosed with rectal cancer, faced a daunting future. Standard treatment meant grueling chemotherapy, radiation, and, in many cases, life-altering surgery that could lead to bowel, urinary, and sexual dysfunction. Some would have needed colostomy bags. Instead of following the usual course, these patients enrolled in a clinical trial to test a new drug.
The results were beyond what anyone anticipated. Every single patient saw their cancer disappear entirely. Physical exams, endoscopies, PET scans, and MRIs found no trace of the disease. It wasn’t just tumor shrinkage or temporary improvement; the cancer was gone.
This post may contain affiliate links. As an Amazon Associate, I earn from qualifying purchases. Read my disclosure policy here.
A cancer diagnosis often brings a problematic journey, harsh treatments, invasive surgeries, and an uncertain future. But for a small group of rectal cancer patients, a clinical trial delivered an outcome no one saw coming. Every single participant walked away cancer-free.
The results left oncologists stunned. Not a trace of cancer remained in scans, not in biopsies, not even under the most rigorous medical scrutiny. Such a complete remission had never been documented in a trial like this.
The Trial That Shocked Experts
Eighteen patients, all diagnosed with rectal cancer, faced a daunting future. Standard treatment meant grueling chemotherapy, radiation, and, in many cases, life-altering surgery that could lead to bowel, urinary, and sexual dysfunction. Some would have needed colostomy bags. Instead of following the usual course, these patients enrolled in a clinical trial to test a new drug.
The results were beyond what anyone anticipated. Every single patient saw their cancer disappear entirely. Physical exams, endoscopies, PET scans, and MRIs found no trace of the disease. It wasn’t just tumor shrinkage or temporary improvement; the cancer was gone.
Dr. Luis A. Diaz Jr. of Memorial Sloan Kettering Cancer Center said, “I believe this is the first time this has happened in the history of cancer.” He co-authored a study in the New England Journal of Medicine, sponsored by GlaxoSmithKline. He noted that he was unaware of any other trial where a treatment eliminated cancer in every patient. Dr. Alan P. Venook, a colorectal cancer specialist at the University of California, San Francisco, said, “A complete remission in every single patient is unheard of.” Although he was not involved in the study, he believed this was the first time such a result had been achieved.
Dostarlimab and Its Role in Cancer Treatment
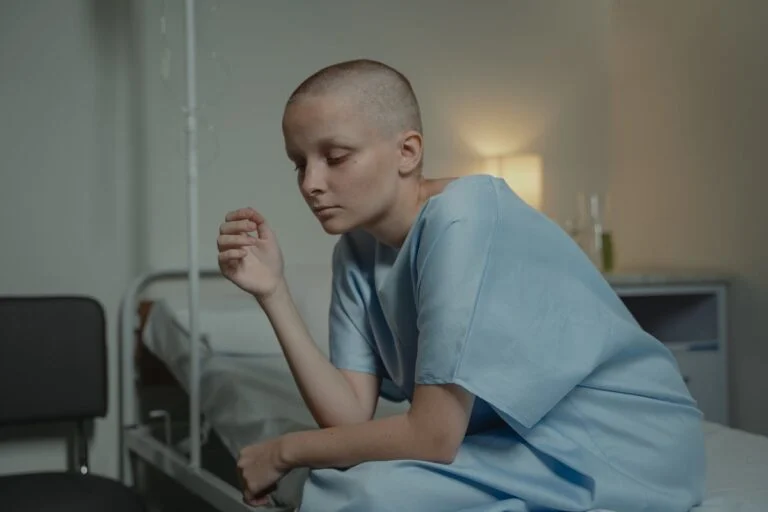
The drug responsible for this medical milestone is dostarlimab, a monoclonal antibody developed by GlaxoSmithKline (GSK). Initially approved for treating endometrial cancer, dostarlimab is now showing groundbreaking potential in rectal cancer treatment. Authors, Hussain and Burney state that “Dostarlimab is a new drug previously used to treat endometrial cancers and has a mechanism of action that is in accordance with other PD-1/PD-L1 inhibitors.
A recent clinical trial has found Dostarlimab to cure 100% of the CRC patients who were given this drug while also showing no adverse events of grade 3 or higher in any patient.” Dostarlimab targets the PD-1 (programmed death-1) receptor, a key component in the immune system’s ability to recognize and attack cancer. Many tumors, including rectal cancer with mismatch repair deficiency (dMMR), use PD-1 to evade immune detection. By blocking this receptor, dostarlimab releases the immune system’s natural defense mechanisms, allowing T-cells to attack and destroy cancer cells. Dostarlimab Works in Cancer Treatment in the following ways:
This post may contain affiliate links. As an Amazon Associate, I earn from qualifying purchases. Read my disclosure policy here.
A cancer diagnosis often brings a problematic journey, harsh treatments, invasive surgeries, and an uncertain future. But for a small group of rectal cancer patients, a clinical trial delivered an outcome no one saw coming. Every single participant walked away cancer-free.
The results left oncologists stunned. Not a trace of cancer remained in scans, not in biopsies, not even under the most rigorous medical scrutiny. Such a complete remission had never been documented in a trial like this.
The Trial That Shocked Experts
Eighteen patients, all diagnosed with rectal cancer, faced a daunting future. Standard treatment meant grueling chemotherapy, radiation, and, in many cases, life-altering surgery that could lead to bowel, urinary, and sexual dysfunction. Some would have needed colostomy bags. Instead of following the usual course, these patients enrolled in a clinical trial to test a new drug.
The results were beyond what anyone anticipated. Every single patient saw their cancer disappear entirely. Physical exams, endoscopies, PET scans, and MRIs found no trace of the disease. It wasn’t just tumor shrinkage or temporary improvement; the cancer was gone.
Dr. Luis A. Diaz Jr. of Memorial Sloan Kettering Cancer Center said, “I believe this is the first time this has happened in the history of cancer.” He co-authored a study in the New England Journal of Medicine, sponsored by GlaxoSmithKline. He noted that he was unaware of any other trial where a treatment eliminated cancer in every patient. Dr. Alan P. Venook, a colorectal cancer specialist at the University of California, San Francisco, said, “A complete remission in every single patient is unheard of.” Although he was not involved in the study, he believed this was the first time such a result had been achieved.
Dostarlimab and Its Role in Cancer Treatment
The drug responsible for this medical milestone is dostarlimab, a monoclonal antibody developed by GlaxoSmithKline (GSK). Initially approved for treating endometrial cancer, dostarlimab is now showing groundbreaking potential in rectal cancer treatment. Authors, Hussain and Burney state that “Dostarlimab is a new drug previously used to treat endometrial cancers and has a mechanism of action that is in accordance with other PD-1/PD-L1 inhibitors.
A recent clinical trial has found Dostarlimab to cure 100% of the CRC patients who were given this drug while also showing no adverse events of grade 3 or higher in any patient.” Dostarlimab targets the PD-1 (programmed death-1) receptor, a key component in the immune system’s ability to recognize and attack cancer. Many tumors, including rectal cancer with mismatch repair deficiency (dMMR), use PD-1 to evade immune detection. By blocking this receptor, dostarlimab releases the immune system’s natural defense mechanisms, allowing T-cells to attack and destroy cancer cells. Dostarlimab Works in Cancer Treatment in the following ways:
- The Body Fights Back: Once unleashed, the immune system eliminates tumors, sometimes leading to complete remission.
2. Cancer Cells Avoid Detection: Normally, tumors suppress immune responses using the PD-1 pathway.
3. Dostarlimab Blocks the PD-1 Defense: This lifts the immune system’s “brakes,” enabling T-cells to recognize and fight cancer.
